Enabling Controlled Substance Sampling
- Browser
- CRM Desktop (Windows)
- iPad
- iPhone
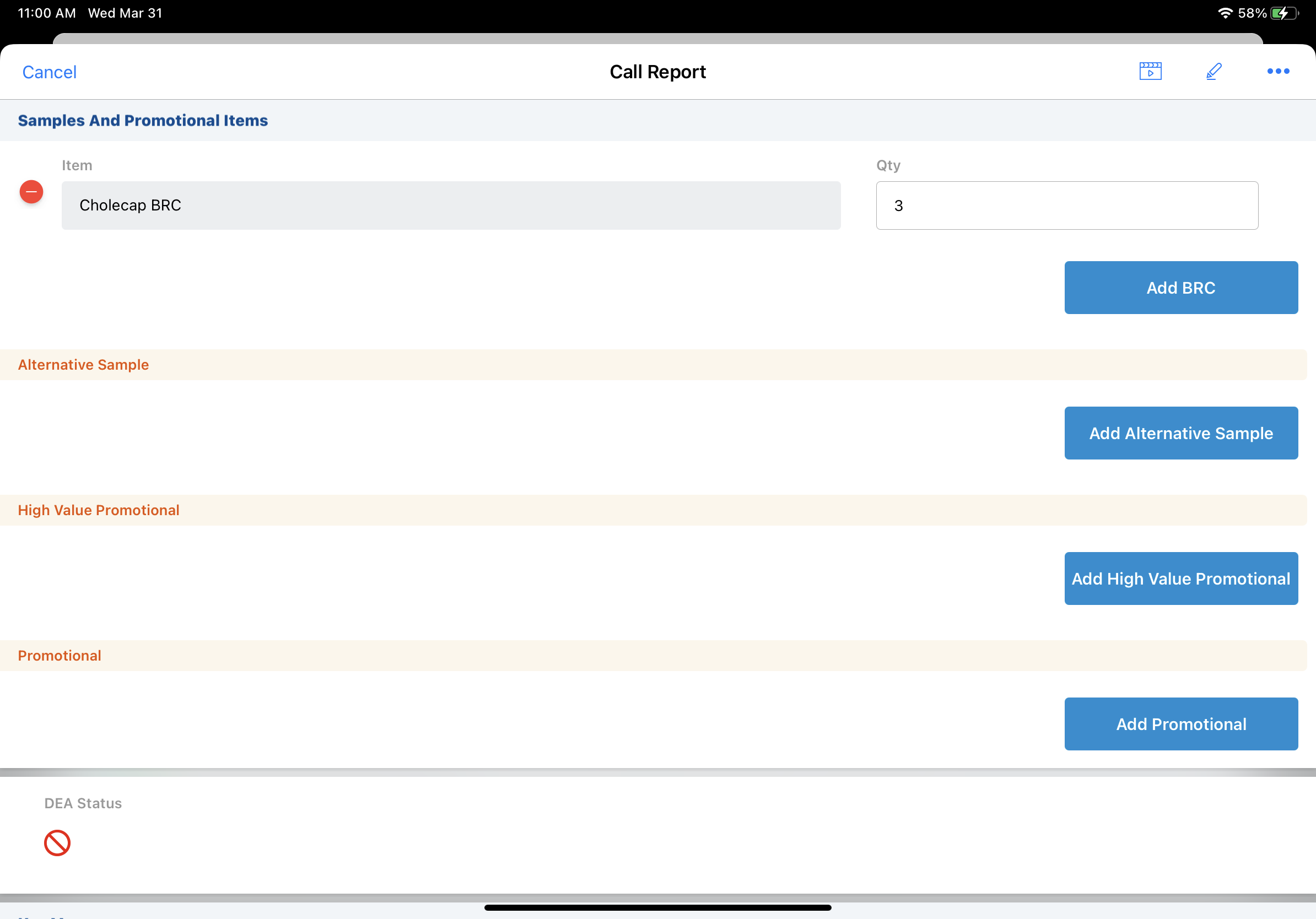
In order to disburse samples or create sample requests/BRCs for controlled substances, users must have access to the appropriate product records, and due to compliance requirements, DEA license information must be included on the call report. DEA license information is stored on the Address_vod object, but it is copied to the call report. Capturing and validating this information on the call report creates a reportable and auditable record of controlled substance sampling and helps ensure disbursements comply with relevant regulations.
For example, Dr. Ackerman requests a sample of a Schedule III product from Sarah Jones. Since Dr. Ackerman’s DEA license information is up to date and his DEA status is Valid, Sarah disburses the requested sample to Dr. Ackerman. She records the disbursement on the call report, captures Dr. Ackerman’s signature, and successfully submits the call report.
Prerequisites
- Configuring Call Sampling
- State Credential Restrictions
In order to receive controlled substance samples, an HCP must have a Sample State Credential Setting record that matches the state and product schedule of the sample being disbursed. Ensure all appropriate HCP accounts have Sample State Credential Setting records for the states where they are licensed to receive controlled substance samples.
Configuring Controlled Substance Product Records
Ensure initial configuration is complete before enabling this functionality.
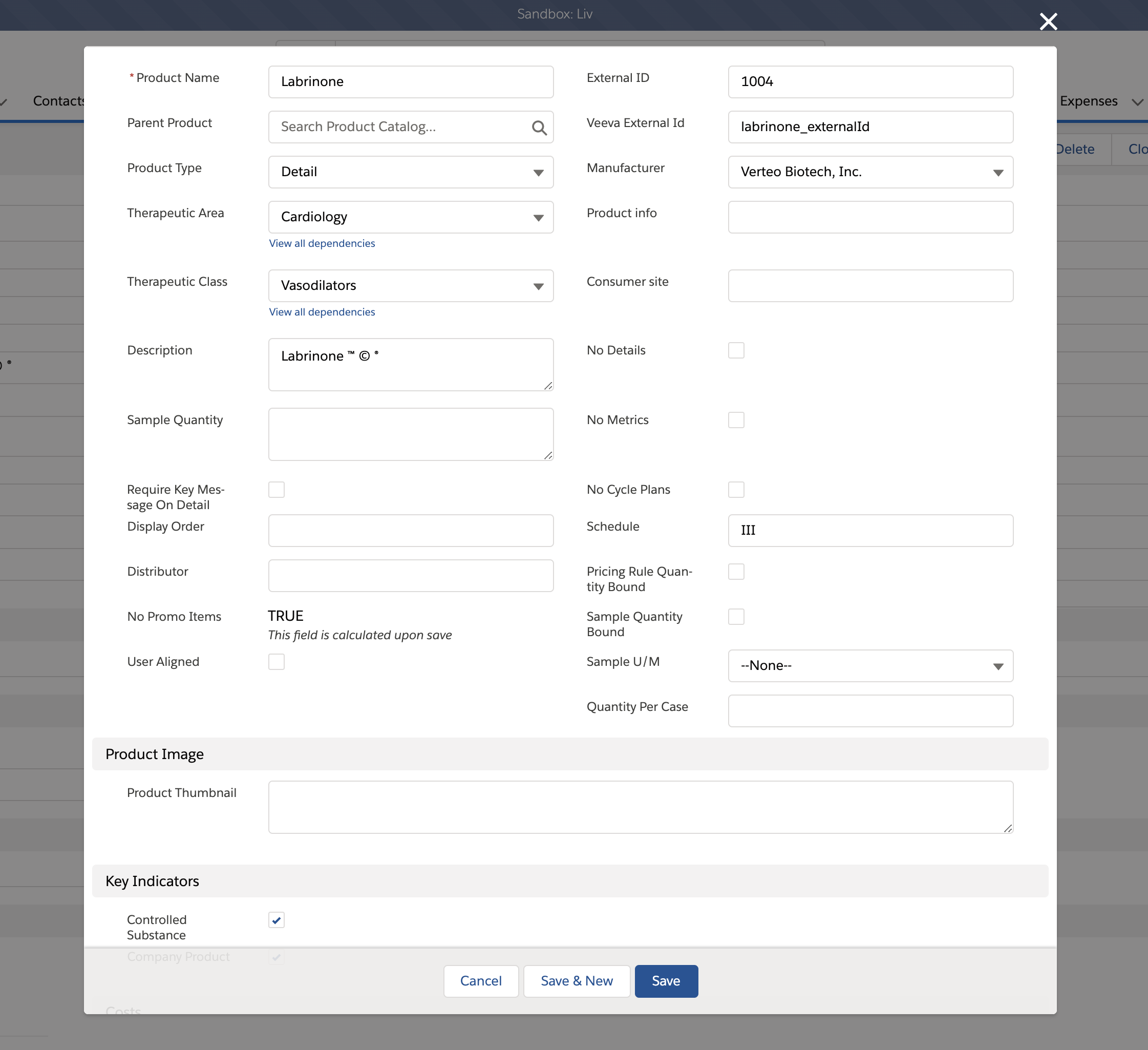
Admins must specifically designate controlled substances in the Product Catalog. To designate a product as a controlled substance:
- Select the appropriate product record.
- Select the Controlled Substance check box on the record to mark the product as a controlled substance.
- Populate the Schedule field on the Product_vod record with the appropriate information for the product.
Text in the Schedule field must be consistent across all areas where DEA schedules are specified. Sample validation requires exact textual matches for this field.
Configuring DEA License Fields
Ensure initial configuration is complete before enabling this functionality.
To capture DEA license information on the call report:
- Ensure sample admins have at least the following permissions (if not already granted in initial configuration):
Object
OLS
Record Types
Fields
FLS
Address_vod
CRUD
Company Maintained
Rep Maintained
- DEA_vod
- DEA_Expiration_Date_vod
- DEA_Schedule_vod
- DEA_Status_vod
Edit
Call2_vod
CRUD
Call_Report_vod - DEA_vod
- DEA_Expiration_Date_vod
- Ship_DEA_vod
- Ship_DEA_Expiration_Date_vod
- zvod_Address_vod__c_DEA_Status_vod (optional)
Edit
Sample_Transaction_vod
R
- Adjustment_vod
- Disbursement_vod
- Receipt_vod
- Return_vod
- Transfer_vod
- DEA_vod
- DEA_Expiration_Date_vod
Edit
Sample_Transaction_Audit_vod
R
n/a
- DEA_vod
- DEA_Expiration_Date_vod
Edit
-
Ensure users have the following permissions (if not already granted in initial configuration):
Object
OLS
Record Types
Fields
FLS
Address_vod
R
- Company Maintained
- Rep Maintained
- DEA_vod
- DEA_Expiration_Date_vod
- DEA_Schedule_vod
- DEA_Status_vod
Read
Call2_vod
CRU
Call_Report_vod - DEA_vod
- DEA_Expiration_Date_vod
- zvod_Address_vod__c_DEA_Status_vod (optional)
Edit
Sample_Transaction_vod
R
n/a
- DEA_vod
- DEA_Expiration_Date_vod
Read
Sample_Transaction_Audit_vod
R
n/a
- DEA_vod
- DEA_Expiration_Date_vod
Read
-
Add the following fields to the appropriate page layouts on the Call2_vod object:
- DEA_vod
- DEA_Expiration_Date_vod
- zvod_Address_vod__c_DEA_Status_vod (optional; enables a DEA status indicator on the call report)
- Select the ENABLE_DUAL_LICENSE_CHECK Veeva Setting check box. This ensures validation runs on both the HCP’s state license and DEA license.
- Populate the following fields on the appropriate accounts’ address records:
- DEA_vod
- DEA_Expiration_Date_vod
- DEA_Schedule_vod
- Populate the DEA_Schedule_vod field with a comma-delimited list of the schedules the account can receive for the designated DEA License
Ensure the text for DEA schedules is consistent across all areas where schedules are specified. In order for an account to receive a product, the schedule associated to the controlled substance sample must be an exact, literal match to one of the schedules listed in the DEA Schedule field.

- Populate the DEA_Schedule_vod field with a comma-delimited list of the schedules the account can receive for the designated DEA License
- DEA_Status_vod
Permission for DEA_License_Address_vod field on the Address_vod object is not required. The DEA Address fields on the call report are copied from Address Line 1, Address Line 2, City, State, Zip, and Zip 4 on the Address record, not from the DEA_License_Address_vod field.
If the address record is locked, only administrators or users with Modify All permissions can edit these fields. (For more information on locking address records, see Using Addresses in Accounts.)
Configuring Ship-To DEA License Information
Users must also capture DEA license information for sample requests/BRCs of controlled substances. To enable sample requests/BRCs of controlled substances:
- Ensure end users creating sample requests/BRCs have the following additional field permissions:
Object
OLS Record Types
Fields
FLS
Call2_vod
CRUD Call_Report_vod - Ship_DEA_vod
- Ship_DEA_Expiration_Date_vod
Edit
-
Add the following fields to the appropriate page layouts on the Call2_vod object:
- Ship_DEA_vod
- Ship_DEA_Expiration_Date_vod
Using Controlled Substance Sampling
If an HCP is licensed to receive controlled substances, users add controlled substance sample products to the call report through the same controls as regular sample products/BRCs. (See Selecting Products for more information.)
Validating DEA License Information
Additional validation helps ensure disbursements of controlled substances comply with the more stringent regulations for controlled substance sampling. In addition to the default validation criteria for Saving a Call with Samples, Validating Samples on Signature Capture, and Submitting a Call with Samples, controlled substance sampling must meet the following requirements:
- The call address/ship-to address and the DEA Address for the selected person account must match exactly
- The DEA license number must follow a specific format, which is enforced via an algorithm check when the account signs for controlled substances
- The signing account’s DEA Status must be set to Valid
- The license’s DEA Expiration Date must be greater than the current date
- The DEA Expiration Date must be valid for the DEA Address
- The schedule associated to the controlled substance sample must be an exact, literal match to one of the schedules listed in the Schedule field on the account’s Address record
- The signing account must have a Sample State Credential Setting record matching their account credentials, the call address/ship-to address state, and the product schedule of any samples/BRCs on the call report
Depending on which Advanced Functionality features are enabled, additional validation criteria apply:
- If the ENABLE_DUAL_LICENSE_CHECK Veeva Setting is enabled, the HCP must have valid state license information for the call addresses’ state
- If Samples State Settings are enabled, the state and delivery mechanism for the sample/BRC cannot be included in the list of restrictions
Swapping the Signee
When controlled substances are on the call report, the Swapping the Signee feature only allows users to select a new signee whose DEA Address information is an exact, literal match to the previous signee’s DEA Address information.
Using the DEA Sample Status Indicator
If the zvod_Address_vod__c_.DEA_Status_vod field is added to the call report page layout, DEA Status indicators display in the appropriate Address picklists and on the call report. This gives users a visual reminder of whether or not the HCP can receive controlled substance samples for the specific address.