What's New In 22R3.3
The CRM 22R3.3 minor release includes several new features, as well as User Visible and Behavior Changes.
Our release notes offer brief, high-level descriptions of enhancements and new features. Configuration is available by selecting the feature name. You can also find a quick overview of 22R3.3 Feature Enablement and Availability information in this release note.
New Fields and New Objects are also included.
Announcements
Ending Support for iOS 14 in 23R1.0
Veeva CRM will no longer support iOS 14 after the 23R1.0 release. Users must upgrade to iOS 15 or higher. Beginning with the 22R3.3 sandbox release on February 23, 2023, for users who are still using iOS 14, a popup displays after signing into Veeva CRM with a message to upgrade devices.
Features
Each new feature is accompanied by a corresponding video. Videos for features in the 22R3.3 release will be available by March 2, 2023.
Approved Email
|
Confirming RSVPs and Activities To prevent systems, for example, bots or firewalls, from confirming Events Management RSVPs or multichannel activities, admins can configure a customized confirmation step for email recipients. This is similar to the confirmation step used for consent confirmation emails. |
|
CLM
|
Fading Out CLM Content on CRM Desktop (Windows) Certain compliance regulations require presentation slides to either display in full or not at all. To help users satisfy these compliance regulations, when the CRM app loses focus due to an overlay or the navigation bar, CLM content remains in the background and fades to black until the overlay or navigation bar is completely closed. |
|
|
|
Navigating Slides and Presentations on CRM Desktop (Windows) Users can navigate between CLM slides and presentations using different controls in the media player, including the arrow buttons and the navigation bar. This allows users to easily and smoothly navigate between any content in their media libraries without requiring navigation to be built into the content itself. On CRM Desktop (Windows), the arrow buttons transition to the previous and next slides, and do not save the slide history. |
|
|
|
Using the Action Menu on CRM Desktop (Windows) Users have access to action menu options directly from the CLM media player, allowing for increased user efficiency during calls. |
|
Events Management
|
Viewing the Next Steps Section in Lightning for Events Management (Sandbox Beta) Orgs using the Lightning for Events Management sandbox beta can view the Next Steps section from an EM_Event_vod record on the Browser (Lightning) platform, enabling event organizers to view guidance for the planning process provided by the event admin. |
|
|
|
Adding Walk-in Attendees in Lightning for Events Management (Sandbox Beta) (Available March 2, 2023) Event organizers in orgs using the Lightning for Events Management sandbox beta can add walk-in attendees to an event. This enables event organizers on the Browser (Lightning) platform to manually enter walk-in information that may have been collected outside of CRM. |
|
|
|
Managing Out of Territory Attendees in Lightning for Events Management (Sandbox Beta) Event organizers in orgs using the Lightning for Events Management sandbox beta can search for attendees outside of their own territory, enabling them to invite these accounts to their events. |
|
Medical
|
Viewing Medical Activities from Vault Clinical in CRM Using the Clinical Operations to Medical CRM Connection, organizations with both a Clinical Operations CTMS Vault and Medical CRM can exchange and view data related to Clinical and Medical activities, and view discussions, and clinical study and site information. This information allows users to have a complete picture of the HCP. |
|
MyInsights Studio
|
Using the Merge Lists Data Element (Available March 2, 2023) Content creators can use the Merge Lists data element to easily combine different types of data in a single display element, such as a chart or table, by merging multiple data elements. The content creator can choose which fields in the input elements are mapped to which fields in the output. Each input maps to exactly one output field. |
|
Territory Management
|
Location Based Targeting in Align Location Based Targeting is a form of sales planning that enables goals to be set against accounts at specific locations, enabling greater specificity in the planning process. Admins can enable Location Based Targeting in Enhanced Territory Feedback to allow end users to view these goals for accounts at specific locations. |
|
User Visible and Behavior Changes
Most new functionality requires some configuration, however users are able to use the following functionality immediately. Select the thumbnail to view a larger image.
CLM
General
| Platform | Description | Before | After |
|---|---|---|---|
| iPad, iPhone | For users who are using iOS 14, a popup displays in Veeva CRM with a message to upgrade their devices to iOS 15 or later before 23R1.0. | n/a | 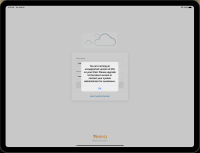
|
22R3.3 Feature Enablement and Availability
New functionality introduced in the Veeva CRM 22R3.3 release is available:
|
CRM Feature Availability and Enablement |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Feature |
UVC |
Configuration Required |
Browser (Classic) |
Browser (Lightning) |
CRM Desktop (Mac) |
CRM Desktop (Windows) |
iPad |
iPhone |
Windows Tablet |
|
Approved Email |
|||||||||
|
No |
Yes |
|
|

|

|
|
|
|
|
|
CLM |
|||||||||
|
No |
No* |

|

|

|

|

|

|
|
|
|
Navigating Slides and Presentations on CRM Desktop (Windows) (parity) |
Yes |
No* |

|

|

|

|

|

|

|
|
Yes |
No* |

|

|

|

|

|

|

|
|
|
Events Management |
|||||||||
|
Viewing the Next Steps Section in Lightning for Events Management (Sandbox Beta) |
No |
Yes |

|

|

|

|

|

|

|
|
Adding Walk-in Attendees in Lightning for Events Management (Sandbox Beta) |
No |
Yes |

|

|

|

|

|

|

|
|
Managing Out of Territory Attendees in Lightning for Events Management (Sandbox Beta) |
No |
Yes |

|

|

|

|

|

|

|
|
Medical |
|||||||||
|
No |
Yes |
|

|

|

|
|
|
|
|
|
Territory Management |
|||||||||
|
No |
Yes |

|

|

|

|
|

|

|
|
* Parity items do not typically require configuration if you are using this functionality on another platform. In some cases, VMOCs must be enabled for the added platform.
|
MyInsights Studio Feature Availability and Enablement |
|
|---|---|
| Feature | Configuration Required |
|
No |
|
New Objects
|
Object Name |
Object Label |
Description |
|---|---|---|
|
Vault_Clinical_Site_vod |
Vault Clinical Site |
Stores Study Site data from Vault Clinical. |
|
Vault_Clinical_Study_vod |
Vault Clinical Study |
Stores Study data from Vault Clinical. |
New Fields
The list below contains all fields added in Veeva CRM 22R3.3. The fields are organized by object. See the Veeva Data Model information for a full listing of Veeva fields.
|
Object |
Field Name |
Field Label |
Description |
Type |
|---|---|---|---|---|
|
Call2_Discussion_vod |
Vault_Clinical_External_Id_vod |
Vault Clinical External ID |
External ID used for connection with Vault Clinical. |
Text |
|
Call2_Discussion_vod |
Vault_Clinical_Site_vod |
Clinical Site |
Specifies the Clinical Site discussed during the Call. |
Lookup |
|
Call2_Discussion_vod |
Vault_Clinical_Study_vod |
Clinical Study |
Specifies the Clinical Study discussed during the Call. |
Lookup |
|
EM_Event_History_vod |
Ending_Stage_vod |
Ending Stage |
The ending stage of the event after lifecycle change. |
Picklist |
|
EM_Event_History_vod |
Starting_Stage_vod |
Starting Stage |
The starting stage of the event when the lifecycle stage changes. |
Picklist |
|
EM_Event_vod |
Stage_Setting_vod |
Stage Setting |
The mapping of event statuses and lifecycle stages. |
Text |
|
EM_Event_vod |
Stage_vod |
Stage |
Current lifecycle stage of an event. |
Picklist |
|
EM_Stage_Configuration_vod |
Event_Rule_vod |
Event Rule |
References the associated event rule. |
Lookup |
|
EM_Stage_Configuration_vod |
Stage_vod |
Stage Name |
Stage for this lifecycle configuration. |
Picklist |
|
EM_Stage_Configuration_vod |
Statuses_vod |
Statuses |
Comma delimited list of event statuses that apply to this event stage. |
Text |
|
Medical_Discussion_vod |
Vault_Clinical_External_Id_vod |
Vault Clinical External ID |
External ID used for connection with Vault Clinical. |
Text |
|
Medical_Discussion_vod |
Vault_Clinical_Site_vod |
Clinical Site |
Specifies the Clinical Site discussed during the Call. |
Lookup |
|
Medical_Discussion_vod |
Vault_Clinical_Study_vod |
Clinical Study |
Specifies the Clinical Study discussed during the Call. |
Lookup |
|
Vault_Clinical_Site_vod |
Country_vod |
Country |
Specifies the country the Clinical Site is within. |
Picklist |
|
Vault_Clinical_Site_vod |
Lifecycle_State_vod |
Lifecycle State |
Lifecycle State of Study Site in Vault Clinical. |
Text |
|
Vault_Clinical_Site_vod |
Principal_Investigator_Lookup_vod |
Principal Investigator |
Specifies the Principal Investigator / Account for the Clinical Site. |
Lookup |
|
Vault_Clinical_Site_vod |
Principal_Investigator_Text_vod |
Principal Investigator |
Specifies the Principal Investigator Name for the Clinical Site. |
Text |
|
Vault_Clinical_Site_vod |
Site_Name_Lookup_vod |
Site Name |
Specifies the Organization / Business Account for the Clinical Site. |
Lookup |
|
Vault_Clinical_Site_vod |
Site_Name_Text_vod |
Site Name |
Specifies the Organization Name of the Clinical Site. |
Text |
|
Vault_Clinical_Site_vod |
Site_Number_vod |
Site Number |
Specifies the site number of the Clinical Site. |
Text |
|
Vault_Clinical_Site_vod |
Vault_Clinical_External_Id_vod |
Vault Clinical External ID |
External ID used for connection with Vault Clinical. |
Text |
|
Vault_Clinical_Site_vod |
Vault_Clinical_Org_External_Id_vod |
Vault Clinical Organization External ID |
External ID used for connection with Vault Clinical. |
Text |
|
Vault_Clinical_Site_vod |
Vault_Clinical_Status_vod |
Vault Clinical Status |
Status of Study Site in Vault Clinical. |
Picklist |
|
Vault_Clinical_Site_vod |
Vault_Clinical_Study_vod |
Study |
Specifies the parent Clinical Study of the Clinical Site. |
Lookup |
|
Vault_Clinical_Study_vod |
Lifecycle_State_vod |
Lifecycle State |
Lifecycle State of Study in Vault Clinical. |
Text |
|
Vault_Clinical_Study_vod |
Protocol_Title_vod |
Protocol Title |
Specifies the full title of the Clinical Study defined in the protocol. |
Text |
|
Vault_Clinical_Study_vod |
Study_Name_vod |
Study Name |
Specifies the short title of the Clinical Study. |
Text |
|
Vault_Clinical_Study_vod |
Study_Phase_vod |
Study Phase |
Specifies the numerical phase of the Clinical Study. |
Text |
|
Vault_Clinical_Study_vod |
Study_Start_Date_vod |
Study Start Date |
Specifies the start date of the Clinical Study. |
Date |
|
Vault_Clinical_Study_vod |
Vault_Clinical_External_Id_vod |
Vault Clinical External ID |
External ID used for connection with Vault Clinical. |
Text |
|
Vault_Clinical_Study_vod |
Vault_Clinical_Product_vod |
Product |
Specifies the Lead Agent Product for the Clinical Study. |
Text |
|
Vault_Clinical_Study_vod |
Vault_Clinical_Status_vod |
Vault Clinical Status |
Status of Study in Vault Clinical. |
Picklist |