Capturing Electronic Signatures for Sampling
- CRM Desktop (Windows)
- iPad
- iPhone
Capturing signatures for sampling on the call report enables users to maintain centralized, comprehensive records of call activities and helps ensure relevant regulatory and compliance requirements are met.
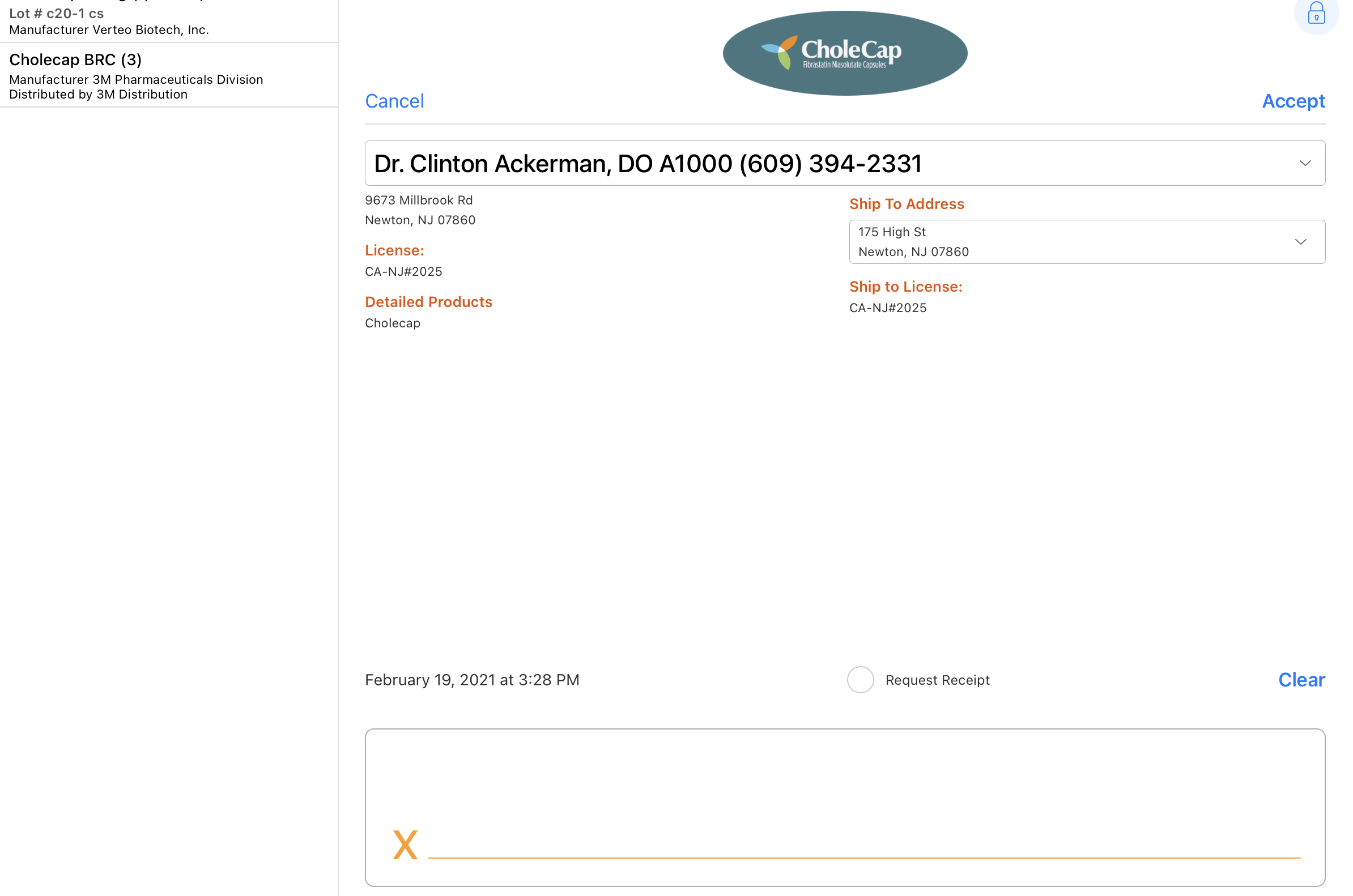
Users can capture electronic signatures for sample disbursements, sample request/BRC, and controlled substances. For example, Sarah Jones details Cholecap to Dr. Ackerman and then disburses a 10 mg sample of Cholecap to him. On the call report, she records the product detailing and disbursement, then presents the electronic signature capture page to Dr. Ackerman. Dr. Ackerman reviews the HCP information, the sample disbursed to him, and the sampling disclaimer, and then signs. Sarah saves the call report with the relevant call details and Dr. Ackerman’s signature.
Ensure initial configuration is complete to use this functionality.
Capturing Signatures Electronically
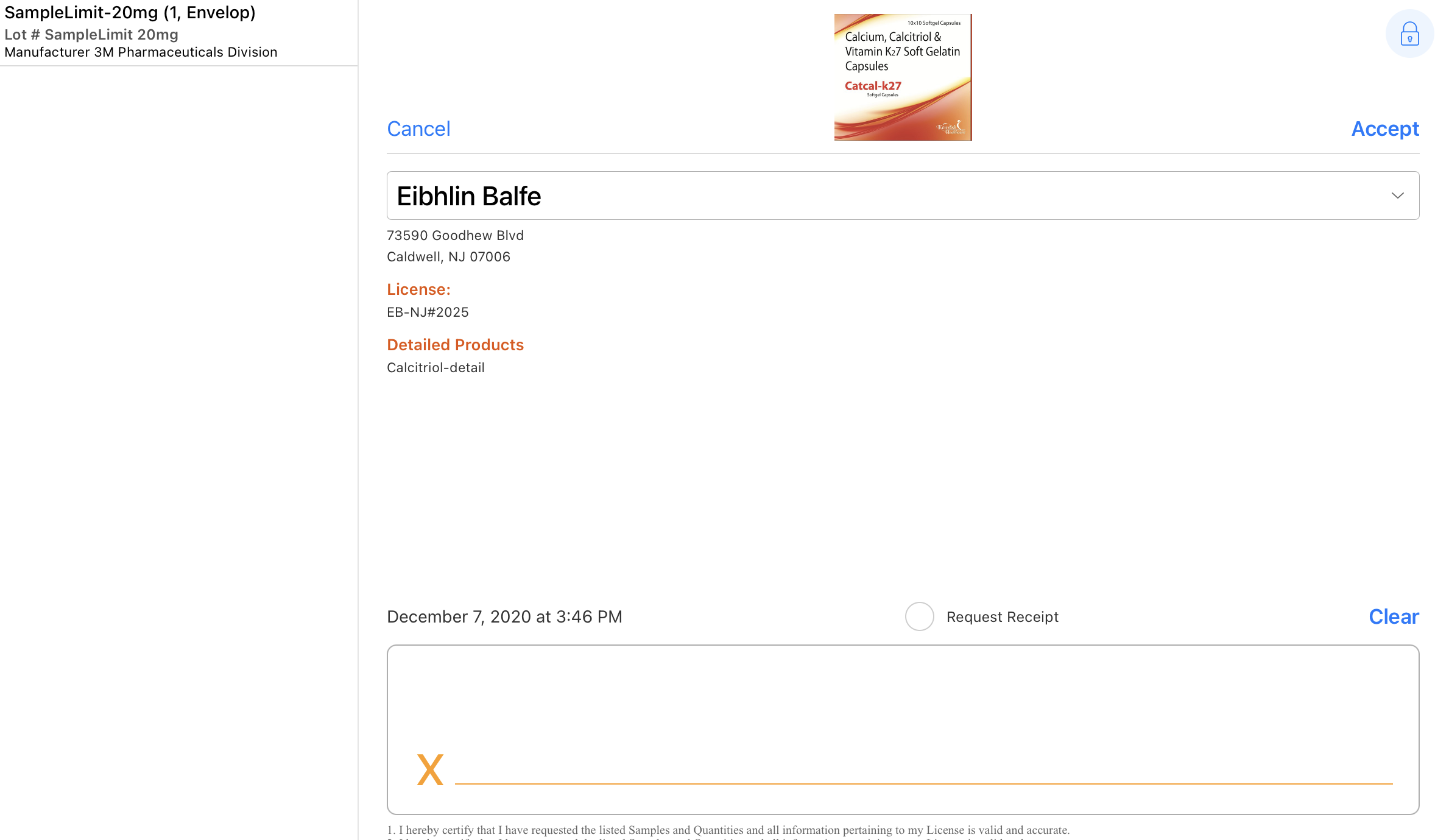
The signature page displays the samples dropped along with the quantity, unit of measure, lot number, and manufacturer/distributor in a bar on the left side of the screen. By default, the Sign button displays only if a sample product or BRC is selected. For group calls with unique activities enabled, the user must also select a sample recipient.
The font size for product names on the signature page displays dynamically based on the length of the product name and related information. The font size does not decrease below 12 pt font.
To capture a signature on the call report:
- Select Sign from the More Actions menu.
- Select the language for the disclaimer (if Displaying Country-Specific Disclaimers is configured).
- Select the padlock icon to hide the Accept and Cancel buttons (optional). Hiding the Accept and Cancel buttons safeguards against the signee dismissing the signature page and accessing confidential information. Selecting in the area of the padlock again restores them.
The HCP does the following on the signature screen:
- Reviews the sample, detail, and disclaimer information on the signature screen.
- Reviews the request receipt information on the signature screen.
- Signs in the Signature box.
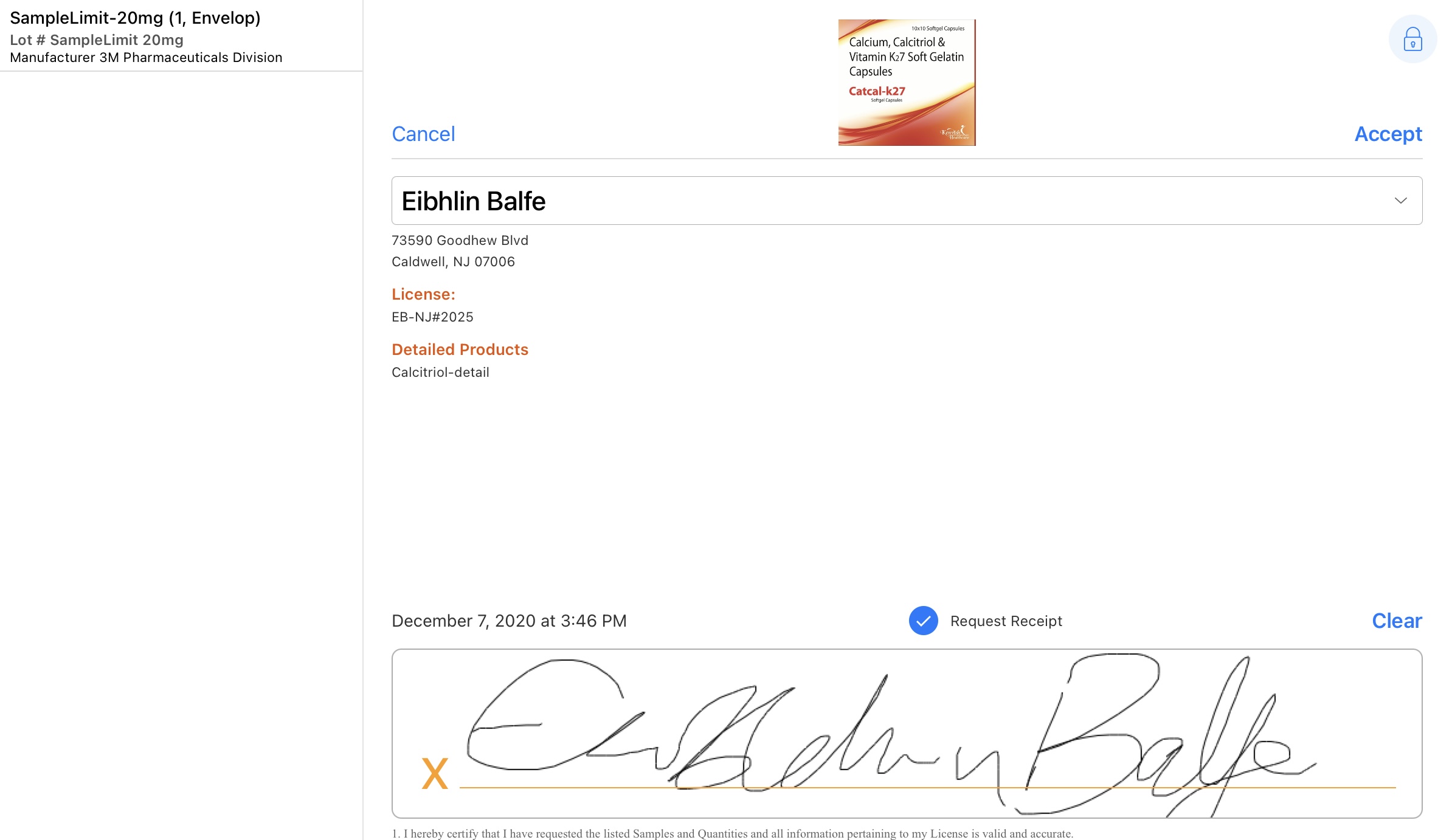
Once the HCP hands the device back to the user, the user:
- Reviews the signature.
- Selects Accept.
The signature can be cleared and re-signed until the user submits the call, unless sample limits are configured and sample products are added to the call report. If sample limits are configured and sample products are on the call report, the signature cannot be cleared and re-signed.
When a signature is captured, the signature datetime automatically overwrites the original call datetime. This helps users keep their schedules accurate without needing to manually update the call datetime after capturing a signature. See Maintaining Separate Call and Signature Datetimes for information on disabling this default behavior.
When the call is submitted, the signature becomes an official record and can no longer be viewed or edited by the user.
Ensuring PDMA Compliance
To comply with Prescription Drug Marketing Act (PDMA) requirements, the following fields are read-only on the signature capture screen:
- HCP Name
- HCP Title [Salutation field]
- Credentials
- License #
- Call Date
- Call Address
- List of all Sample Lots disbursed including:
- Quantity
- Sample Name
- Lot #
- Manufacturer
- Distributed By
- Disclaimer
Locking Sample and BRC Information
To ensure accuracy and regulatory compliance, the following fields on the Call2_vod object are locked when users save a signature on a call with samples, alternative sample, high value promotional items, or BRCs:
- RecordTypeId
- Account_vod
- Address_vod
- Call_Datetime_vod
- Call_Date_vod
- Sample_Card_vod
- Sample_Send_Card_vod
- Supervising_Physician_vod
- All fields placed in the zvod_samples_vod section on the call report
The same fields are locked after users submit Paper-Based Sampling calls with samples, alternative samples, high value promotional items, or BRCs, and a sample card number or sample send card number.
Signature Length Requirements
The storage limit for the Signature_vod field on Calls, Medical Inquiries, and Medical Events is 32k. If an end user tries to save a signature that is too short or too long, an error message displays indicating the signature is not valid.
The captured signature must have at least three data points and at least 100 pixels in length. For reference, a single straight line is two data points.
Considerations
If the ENABLE_SAMPLE_DISTRIBUTED_BY_SIG Veeva Setting is enabled and the Company name is populated, the signature page displays the user's company name as the distributor when users capture a signature for sample products only.
The ENABLE_SAMPLE_DISTRIBUTED_BY_SIG Veeva Setting does not apply to BRC products. For BRC products, the distributor is always the Distributor_vod field value from the BRC's Product Catalog record.
Platform Specific Details
iPhone
To capture a signature for sample disbursements on iPhone:
- Select Sign from the More Actions menu.
- Select the language for the disclaimer (if Disclaimer languages are configured).
- Select the Request Receipt email address (if Approved Email Receipts for Signature Transactions is configured and an email receipt is desired).
- Select Start to begin the signature capture process.
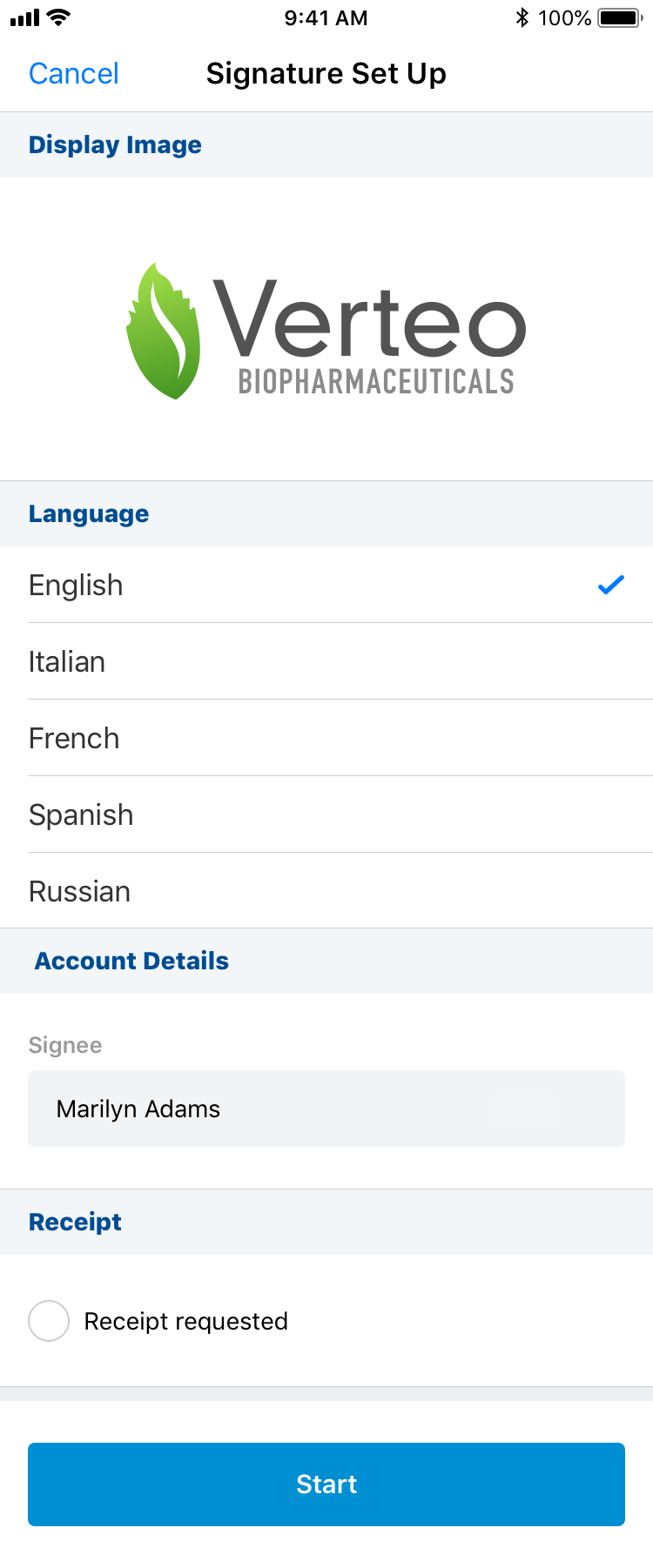
After the signature screen launches, the HCP:
- Reviews the signature details, then selects Next if the account and sample information is accurate.
- Reviews the disclaimer and request receipt information, then select Next to launch the signature screen.
- Signs in the Signature box.
- Selects Accept. The hand over screen displays.
If the HCP selects Back, the screen locks and displays an error message.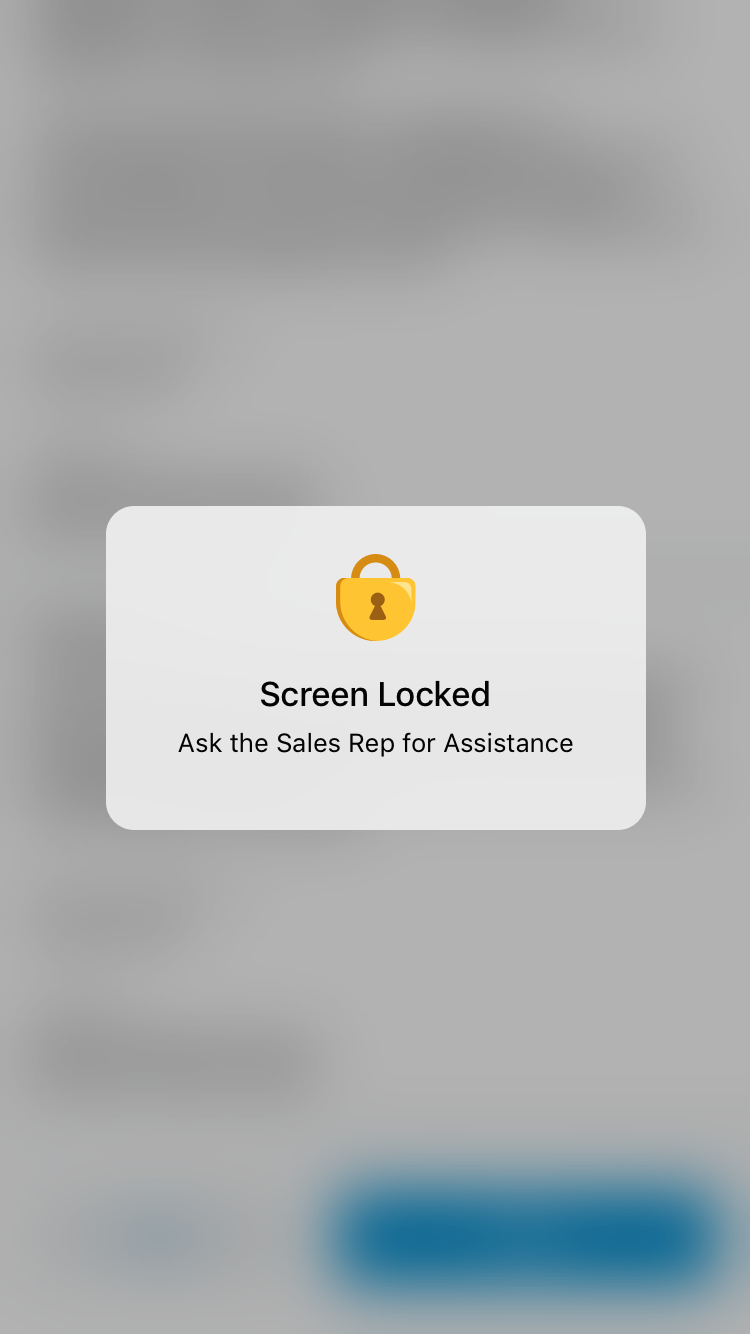
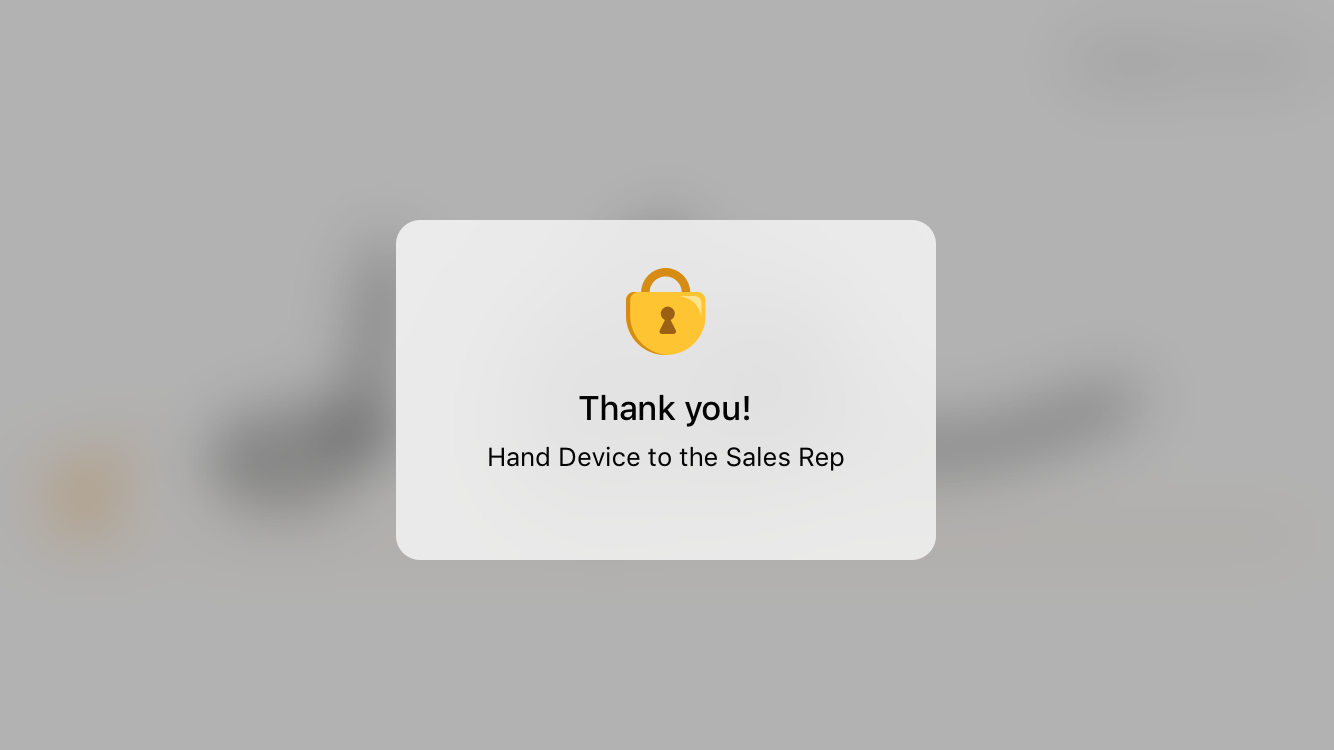
Once the HCP hands the device back to the user, the user:
- Reviews the signature.
- Selects Done.
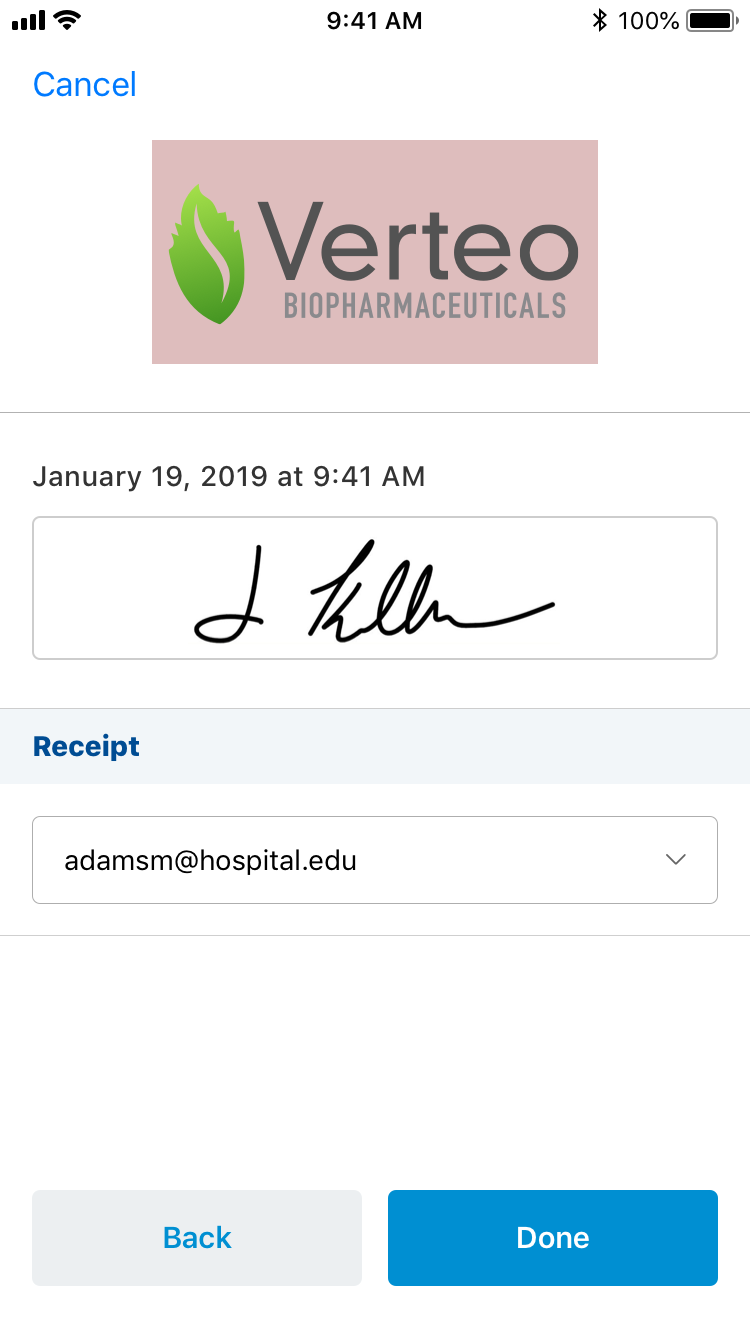
When the user selects Done, the Signature page is locked and all fields are stamped in the audit trail. The signature cannot be canceled or edited.